Реферат: The effect of light intensity on the amount of chlorophyll in “Cicer arietinum”
The plants are naturally blocked in the conversion of protochlorophyllide to chlorophyllide. In normal plants these results in accumulation of a small amount of protochlorophyllide which is attached to holochrome protein. In vivo at least two types of protochlorophyllide holochrome are present. One, absorbing maximally at approximately 650 nm, is immediately convertible to chlorophyllide on exposure to light. If ALA is given to plant tissue in the dark, it feeds through all the way to protochlorophyllide, but no further. This is because POR, the enzyme that converts protochlorophyllide to chlorophyllide, needs light to carry out its reaction. POR is a very actively researched enzyme worldwide and a lot is known about the chemistry and molecular biology of its operation and regulation. Much less is known about how POR works in natural leaf development.
![]() ALA Portoporphyrine
ALA Portoporphyrine
Protochlorophyllide
Chlophyllide
![]()
![]() Chlorophyll b Chlorophyll a
Chlorophyll b Chlorophyll a
Chlorophyll[3] is a green compound found in leaves and green stems of plants. Initially, it was assumed that chlorophyll was a single compound but in 1864 Stokes showed by spectroscopy that chlorophyll was a mixture. If dried leaves are powdered and digested with ethanol, after concentration of the solvent, 'crystalline' chlorophyll is obtained, but if ether or aqueous acetone is used instead of ethanol, the product is 'amorphous' chlorophyll.
In 1912, Willstatter et al. (1) showed that chlorophyll was a mixture of two compounds, chlorophyll-a and chlorophyll-b :

Chlorophyll-a (C55 H72 MgN4 O5 , mol. wt.: 893.49). The methyl group marked with an asterisk is replaced by an aldehyde in chlorophyll-b (C55 H70 MgN4 O6 , mol. wt.: 906.51).
The two components were separated by shaking a light petroleum solution of chlorophyll with aqueous methanol: chlorophyll-a remains in the light petroleum but chlorophyll-b is transferred into the aqueous methanol. Cholorophyll-a is a bluish-black solid and cholorophyll-b is a dark green solid, both giving a green solution in organic solutions. In natural chlorophyll there is a ratio of 3 to 1 (of a to b ) of the two components.
The intense green colour of chlorophyll is due to its strong absorbencies in the red and blue regions of the spectrum, shown in fig. 1. (2) Because of these absorbencies the light it reflects and transmits appears green.
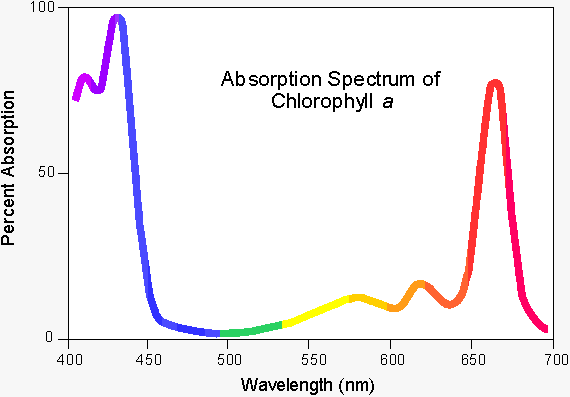
Fig. 1 - The uv/visible adsorption spectrum for chlorophyll.
Due to the green colour of chlorophyll, it has many uses as dyes and pigments. It is used in colouring soaps, oils, waxes and confectionary.
Chlorophyll's most important use, however, is in nature, in photosynthesis. It is capable of channelling the energy of sunlight into chemical energy through the process of photosynthesis. In this process the energy absorbed by chlorophyll transforms carbon dioxide and water into carbohydrates and oxygen:
CO2 + H2 O ![]() (CH2 O) + O2
(CH2 O) + O2
Note: CH2 O is the empirical formula of carbohydrates.
The chemical energy stored by photosynthesis in carbohydrates drives biochemical reactions in nearly all living organisms.
In the photosynthetic reaction electrons are transferred from water to carbon dioxide, that is carbon dioxide is reduced by water. Chlorophyll assists this transfer as when chlorophyll absorbs light energy, an electron in chlorophyll is excited from a lower energy state to a higher energy state. In this higher energy state, this electron is more readily transferred to another molecule. This starts a chain of electron-transfer steps, which ends with an electron being transferred to carbon dioxide. Meanwhile, the chlorophyll which gave up an electron can accept an electron from another molecule. This is the end of a process which starts with the removal of an electron from water. Thus, chlorophyll is at the centre of the photosynthetic oxidation-reduction reaction between carbon dioxide and water.
Treatment of cholorophyll-a with acid removes the magnesium ion replacing it with two hydrogen atoms giving an olive-brown solid, phaeophytin-a . Hydrolysis of this (reverse of esterification) splits off phytol and gives phaeophorbide-a . Similar compounds are obtained if chlorophyll-b is used.

Chlorophyll can also be reacted with a base which yields a series of phyllins, magnesium porphyrin compounds. Treatment of phyllins with acid gives porphyrins.

Now knowing all these factors affecting the synthesis and destruction of chlorophyll I propose that the amount of chlorophyll in plant depends on light intensity in the following way: with the increase of light intensity the amount of chlorophyll increases, but then it starts decreasing because light intensity exceed the point when there is more chlorophyll destructed than formed.

|
|
|

|
|

Variables.