Лабораторная работа: Measuring specific latent heat of vaporization of water
Introduction:
The aim of this experiment was to determine the specific latent heat of vaporization of a liquid using basic equipment and calculations. The accepted value of the latent vaporization heat is 2.3x 104 Jkg-1 . Hopefully the results obtained will be similar to the accepted value.
Apparatus:
Kettle
3 beam Scales
Stop watch
Variables:
Method:
1. Take the kettle and fill it up half way with water
2. Put it on the scales and measure the mass
3. Record the mass in the data table
4. Turn on the kettle
5. When the water starts to boil start timing
6. Stop timing when 50 grams of water have been lost
7. Record in the data table
8. Repeat steps 1-7 five times
Data Collection and Analysis
Time of the trials
| Trials | Mass/kg ± 0.005 | Time/s ± 5s |
| 1 | 0.050 | 187 |
| 2 | 0.050 | 180.0 |
| 3 | 0.050 | 180.0 |
| 4 | 0.050 | 192 |
| 5 | 0.050 | 145 |
| Average | 0.050 | 186 |
Uncertainties
The scientist determined the uncertainties by measuring them. The scientist wasn’t sure when to start timing the boiling water, and as there was no set time time written in the procedure , so the uncertainty was determined to be 5s. As the scales used to measure the mass of the water were not electronic, but three beam, the minimum uncertainty was determined to be 5 grams . The uncertainty for the specific latent heat of vaporization was based on the uncertainties of mass and time.
Pt=mLv 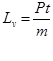
Trial 1 m=0.05 t=187s
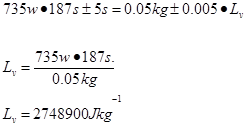
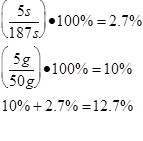
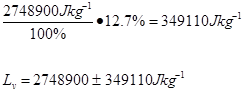
Trial 2 m=0.05 t=180s
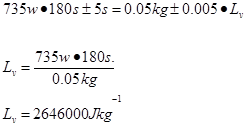
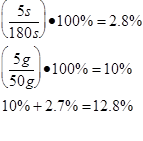
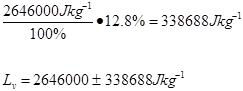
Trial 3 m=0.05g t=180s
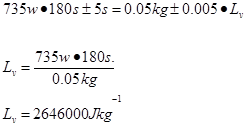
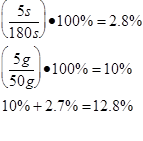
--> ЧИТАТЬ ПОЛНОСТЬЮ <--